How Does Yeast Metabloize
Posted : admin On 10.10.2019A: Yeast metabolizes sugar by splitting the six-carbon sugars into two molecules of pyruvate, then splitting the pyruvate molecules by removing carbon dioxide from them, and finally adding hydrogen ions to create ethanol molecules. This process is known as fermentation, and it. How can the answer be improved?
Longitudinal StudySED 695B; Fall 2005Research Question:Students are often confused by the term isomer. Eventually, they memorize a definition and know that isomers share atomic composition, but vary in their structures. What are the consequences for organisms? Can organisms use any molecule for energy as long as they have the same chemical formulas? Or does the structure of each molecule affect the usefulness of a molecule? In this investigation, it is determined that not all sugar is the same.
Only certain configurations of sugar molecules can be used by yeast. Three Monomers Shown Below-Three Dimers Shown AboveA Possible SetupStandards addressed:Biology/Life SciencesCell Biology1.
The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism’s cells. As a basis for understanding this concept:b. Students know enzymes are proteins that catalyze biochemical reactions without altering the reaction equilibrium and the activities of enzymes depend on the temperature, ionic conditions, and the pH of the surroundings.g. Students know the role of the mitochondria in making stored chemical-bond energy available to cells by completing the breakdown of glucose to carbondioxide.h. Students know most macromolecules (polysaccharides, nucleic acids, proteins,lipids) in cells and organisms are synthesized from a small collection of simpleprecursors.Investigation and Experimentation1. Scientific progress is made by asking meaningful questions and conducting careful investigations.
As a basis for understanding this concept and addressing the content in the other four strands, students should develop their own questions and performinvestigations. Students will:d. Formulate explanations by using logic and evidence.
Procedures:. Fill the six eudiometers with colored tap water (colored water is easier to read). Invert each in a 600mL or larger beaker on a ring stand. Create 10% solutions of each of the six sugars. (add 10.0g sugar to 90.0mL purified water) each in a 250mL Erlenmeyer flask.
Label each flask. Assemble #5 stoppers with glass and rubber tubing. Measure six 1.0g portions of yeast.
Place each portion in a different flask. Install the stopper apparatus into each test tube. Insert the unattached end of each rubber tube into the corresponding inverted eudiometer. Start the clock. Sugars are simple carbohydrates.
Also called saccharides, the come in two forms: monosaccharides and disaccharides. Monosaccharides have the chemical formulaand Disaccharides have the chemical formula.However, many different configurations exist for each of the two kinds. These different configuration of atoms are called isomers.
Isomers of sugars are important to life because organisms have evolved various enzymes to access the energy in each form. Some organisms are therefore better at getting at some forms of sugar than other forms because of the enzymes that they can use.
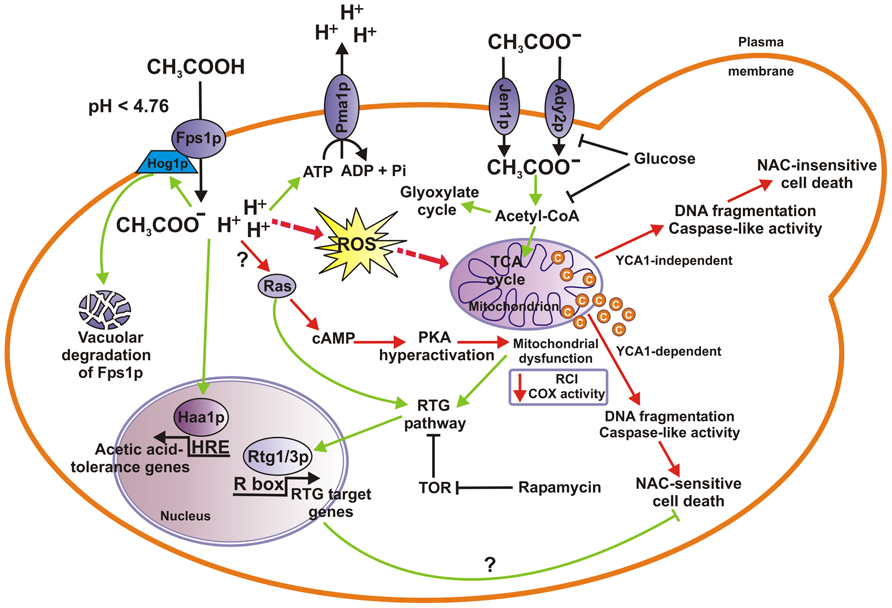
. The principal knowledge of the metabolic capabilities will help us understand the peculiarities that yeast reveals in the breakdown of organic compounds, production of new cell‐specific components, and generation of energy necessary in anabolic pathways. First, we consider the major sources for energy production in S. Cerevisiae – the hexose carbon compounds. Since this yeast (as well as many others) can adapt its metabolism to aerobic or aerobic conditions, we have to differentiate between respiration and oxidative phosphorylation, on the one hand, and alcoholic fermentation, on the other hand. In this context, we describe the effects of glucose repression and diauxie.
The possibilities of how yeast utilizes other hexose sugars, non‐hexose carbon sources, or complex carbon sources are outlined. Gluconeogenesis and carbohydrate biosynthesis are explained in view of yeast's potential to store different forms of carbohydrate for retrieval of energy. Following this, we deal with the utilization and manufacturing of “unusual” hexoses and amino sugars that play an important role in the biosynthesis of cell‐specific macromolecules. A particular section is devoted to yeast compounds that contain inositol as a constituent, such as InsPs and the various phosphatidylinositol derivatives. The regular order of post‐translational N‐ and O‐linked glycosylation of proteins is presented in some detail.
Similar attention is given to the structural carbohydrates that have an outstanding role in yeast cell wall organization. Next, we consider fatty acid and lipid metabolism, which in yeast reveals some specific features. In discussing the glycolipids, we focus on sphingolipids and GPI, which latter have a dominant role as lipid membrane anchors. This section includes a survey of the isoprenoid derivatives, which are particularly synthesized and utilized in yeast.
Yeast Metabolism Journal
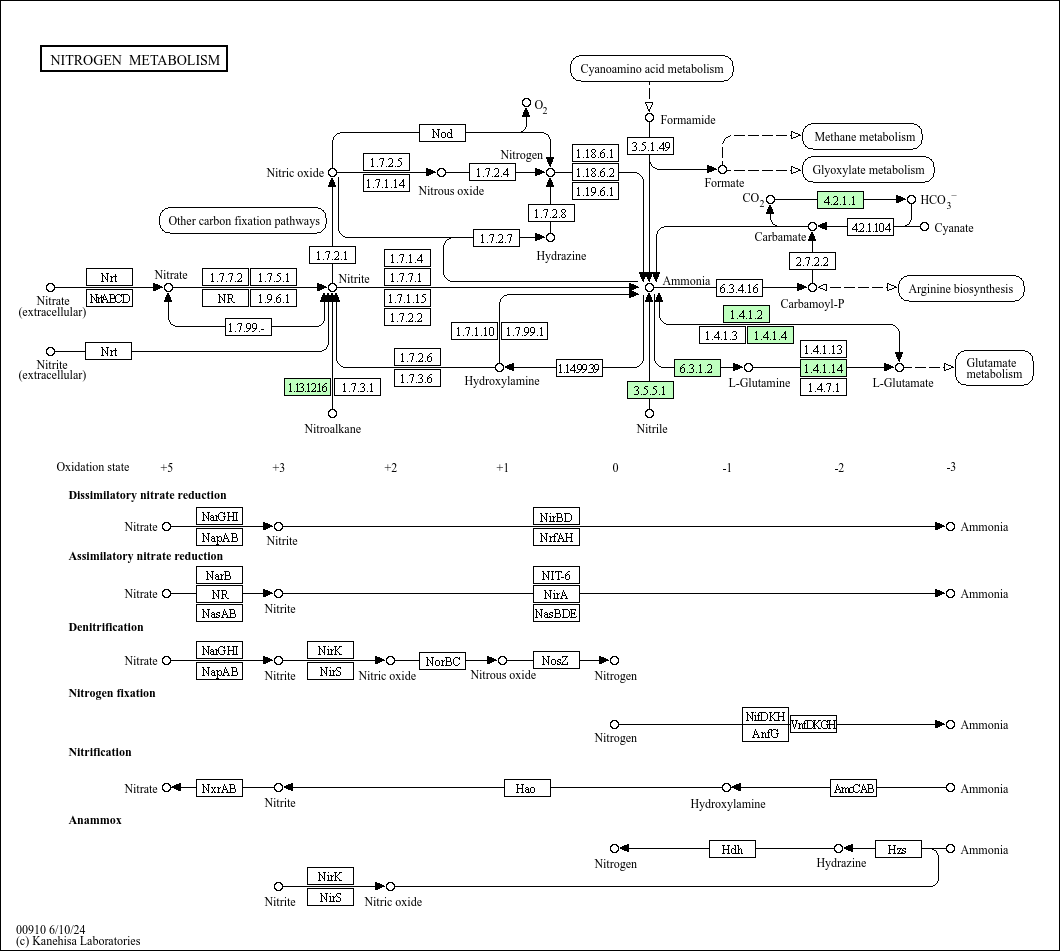
Nitrogen metabolism considers the utilization of organic and inorganic sources in catabolic pathways, whereby the fact that yeasts can live on ammonium as a sole nitrogen source comes as a real surprise. Employing urea as a nitrogen source is restricted to yeast species other than S. Yeast has the capacity to biosynthesize virtually all amino acids from simple carbon sources plus a nitrogen source, assimilation of sulfur from sulfate for the few sulfur‐containing amino acids (cysteine, methionine, homocysteine), together with constituents from some cofactors. One of the most important activities in nitrogen metabolism concerns protein biosynthesis.
We do not present a detailed picture in this overview, but point out further reading/references pertinent to this extremely important field. The following section then presents a concise overview of the manufacturing and breakdown of nucleotide compounds in yeast, most of whose features are common to all organisms. Except in degradation pathways, there are some unusual aspects in fungi. We add a description of nucleotide‐modifying enzymes, which have been studied in S. Cerevisiae in great detail. Sections dealing with the metabolism of phosphorus (phosphate) and sulfur in yeast as well as the capabilities of yeast to synthesize most of its “vitamins” and cofactors from endogenous sources complement our metabolic excursion.